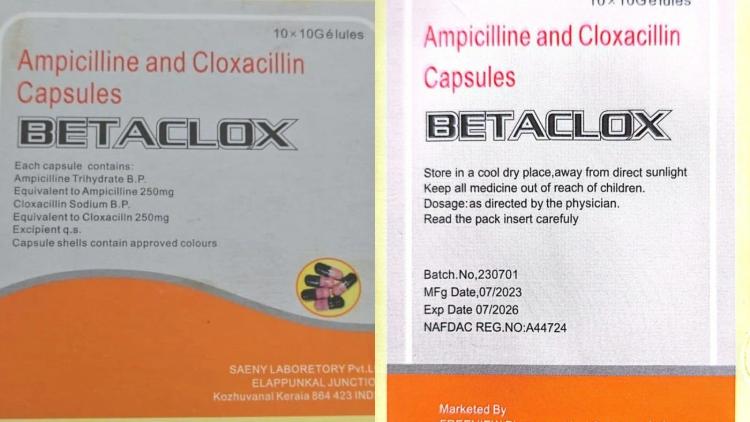
The National Agency for Food and Drug Administration and Control (NAFDAC) has raised an alarm over the circulation of a suspected fake antibiotic, BETACLOX (Ampicillin 250mg and Cloxacillin 250mg), in Nigeria’s drug market.

According to the agency, the falsified product carries fraudulent registration details and originates from an unverified source, posing a serious danger to public health.
The warning followed a report from a retail outlet in Zaria, Kaduna State, which discovered the suspicious product after purchasing it from a distributor in Kano.

Investigations revealed that the NAFDAC registration number (A4-4724) printed on the fake BETACLOX actually belongs to Mebendazole 500mg, manufactured by Chi Ltd., confirming a case of number misappropriation.

The falsified product was allegedly imported by Freeview Pharmaceutical Ltd., with an address listed as 128 MCC Road, Calabar, but verification by NAFDAC showed that the genuine company is located at 101 MCC Road, Calabar, exposing the fake claim.

Details of the falsified product:
Product Name: BETACLOX (Ampicillin 250mg and Cloxacillin 250mg)
Batch No.: 230701
Manufacturer: Saeny Laboratory Pvt. Ltd., Kerala, India
Manufacturing Date: 07/2023
Expiry Date: 07/2026
Fake NAFDAC Reg. No.: A4-4724
NAFDAC has directed all zonal directors and state coordinators to intensify surveillance and remove the fake drug from circulation.

The agency urged pharmacists, distributors, and healthcare professionals to source drugs only from authorised suppliers and to scrutinise packaging for authenticity.
Members of the public are encouraged to report suspicious medicines to the nearest NAFDAC office or through the agency’s toll-free lines.