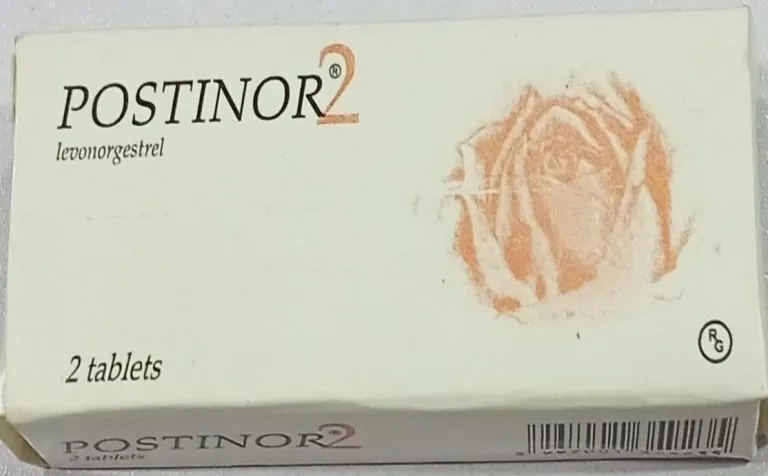
The National Agency for Food and Drug Administration and Control (NAFDAC) has raised an alarm over the circulation of counterfeit batches of Postinor-2 (Levonorgestrel 0.75mg) emergency contraceptives in Nigeria.
In a statement released on Monday, the agency revealed that the falsified products, labeled as Type 1 and Type 2 batches, were flagged after the Society for Family Health (SFH) — the authorised importer — confirmed it did not bring those consignments into the country.
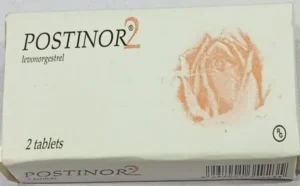
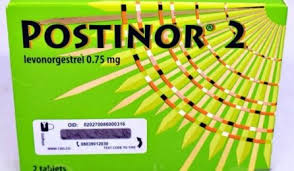
According to NAFDAC, the fake products carry noticeable errors on their packaging, including a misspelt verification sticker reading “Veify” instead of “Verify” and the phrase “Distnibuted in Nigeria” instead of “Distributed in Nigeria.” The original packs, the agency emphasized, have clearer fonts and correct spellings.

“The National Agency for Food and Drug Administration and Control (NAFDAC) hereby notifies the public of falsified Type 1 and 2 batches of Postinor-2 (Levonorgestrel 0.75mg) product in circulation,” the agency stated.
NAFDAC urged Nigerians to carefully inspect Postinor-2 packs before use, purchase only from licensed pharmacies, and report suspicious products to the agency for verification.

The agency warned that using counterfeit emergency contraceptives poses serious health risks, including the possibility of unwanted pregnancies and other complications, and called on the public to stay vigilant while it works with law enforcement to track and remove the falsified products from circulation.